Title
題目
Upper-body free-breathing Magnetic Resonance Fingerprinting applied tothe quantification of water T1 and fat fraction
用于水T1值和脂肪分數量化的上半身自由呼吸磁共振指紋成像?
01
文獻速遞介紹
磁共振指紋成像(MRF)是十年前推出的一種高效成像范式,可實現多種MRI參數的快速同步量化(Ma等,2013)。MRF的核心原理是采集一系列高度欠采樣圖像,隨后逐像素與預先計算的元素字典(即“指紋”)進行擬合,以提取潛在的組織參數。在MRF可測量的眾多參數中,脂肪分數(FF)、水的T1值(𝑇1𝐻2𝑂)、水的T2值(𝑇2𝐻2𝑂)和/或脂肪的T1值(𝑇1𝑓𝑎𝑡)已在多個解剖部位被證實為極具潛力的影像生物標志物,包括心臟(Jaubert等,2020c,2021)、肝臟(Jaubert等,2020a;Velasco等,2022)和骨骼肌(Cencini等,2019;Marty和Carlier,2020;Ostenson等,2019)。例如,Marty和Carlier(2020)提出的MRF T1-FF序列已被用于骨骼肌組織的𝑇1𝐻2𝑂和FF定量監測。該方法已在3T磁場下應用于不同神經肌肉疾病患者的下肢肌肉,能夠聯合重建𝑇1𝐻2𝑂、𝑇1𝑓𝑎𝑡、FF、B0和B1圖(Marty等,2021b;Gerhalter等,2021;Fromes等,2023)。然而,由于生理運動(尤其是呼吸運動)的影響,在上半身測量這些參數面臨諸多挑戰。 具體而言,呼吸肌(如肋間肌和膈肌)屬于薄組織結構,在神經肌肉疾病中常受累及。患者往往會出現明顯不適,有時甚至需要通氣支持。盡管已采用超聲、脂肪飽和T2加權MRI等定性成像技術(Vieira Santana等,2020;Cicero等,2020)以及MR肺活量測定等功能成像技術(Harlaar等,2022)對這些關鍵目標進行研究,但由于呼吸運動帶來的挑戰,能夠在功能障礙顯現前早期識別結構異常的定量MRI尚未開展。 在定性笛卡爾MRI中,呼吸運動常導致相位編碼方向出現模糊和偽影(Wood和Henkelman,1985)。在非笛卡爾MRI中,盡管k空間中心經多次采樣可減少偽影(Glover和Pauly,1992),但模糊問題依然存在。早期觀察表明,運動引起的偽影對定量MRI影響顯著,會導致參數估計偏差(Giri等,2012)。 因此,學界提出了多種方法以減輕MRI中的呼吸運動影響。通常,第一步是在采集過程中檢測運動,將數據劃分為多個呼吸時相。這可通過多種方式實現,例如將用于測量腹腔擴張與收縮的呼吸波紋管置于患者腹部周圍(Ehman等,1984)。但該技術的運動檢測準確性可能受波紋管放置位置和患者呼吸強度影響(Santelli等,2011)。近期開發的Pilot Tone設備通過發射固定頻率的射頻波(該頻率超出MRI采集帶寬)解決了這一問題(Solomon等,2021)。這種射頻波會受到多種運動(主要是呼吸運動)的調制。Pilot Tone的優勢在于其在磁體孔內的放置靈活,對患者的依賴性較低。 另一種方法是在MRI序列中集成門控輻條(或導航器),按精確時間間隔沿特定方向采集身體的低分辨率圖像(Ehman和Felmlee,1989)。隨后可追蹤肺-肝界面等解剖結構的運動,將數據分類至不同呼吸時相。后一種方法與MRI序列本身同步,無需外部設備。 將采集的數據分入不同呼吸時相后,可采用多種策略進行運動校正。第一種方法是保留單一呼吸時相的數據(通常為完全呼氣時相,因其持續時間往往長于其他時相)(Huang等,2021)。但這種方法會丟棄大量數據,可能引發欠采樣問題。一種前瞻性解決方案是延長MRI采集時間,以收集足夠數據重建所需呼吸時相(Ehman等,1984)。盡管如此,該方法不可避免地會延長掃描時間。為利用所有呼吸時相的數據,回顧性運動校正(MoCo)方法應運而生。 部分方法直接在k空間中進行運動校正,例如自動聚焦法,其通過應用相位校正來最小化圖像梯度熵(Atkinson等,1997)。但該方法主要適用于校正剛性變形或分段剛性變形,需對每個線圈的k空間單獨校正(Cheng等,2012)。對于自由形式的變形,首選在圖像空間中進行校正。一種方法是通過引入低秩約束(如多任務處理)同時重建所有運動狀態的圖像(Christodoulou等,2018)。這些方法的優勢在于無需估計不同狀態間的運動場。但最小化問題的維度可能較大,且可能存在不適定性,因此難以適用于高分辨率3D成像。另一種方法是先在低分辨率圖像上估計變形場,再應用該場將所有圖像配準至同一參考圖像。定性MRI中已提出高維總變分運動校正(MoCo-HDTV)等方法(Rank等,2017),通過迭代估計變形場和重建圖像,并在每一步后逐步提高空間分辨率,來重建受運動干擾的圖像。針對1.5T的MRF,Cruz等(2022)提出利用來自不同運動狀態的去噪單個體積估計變形場,隨后將該變形場納入單個體積的最小二乘重建中,以便在模式匹配后生成MRF圖。然而,3T磁場下視野內更大的B1變化,加之MRF T1-FF序列的徑向采集方案導致的欠采樣偽影增加,使得獲取質量足夠的運動分辨單個體積用于圖像配準面臨挑戰。 在本研究中,我們提出一種新穎的運動校正MRF T1-FF(MoCo MRF T1-FF)方法,通過優化的初步運動掃描迭代估計運動場,并在模式匹配前應用該運動場校正MRF采集數據,以重建上半身區域的運動校正參數圖。從方法學角度而言,該方法有兩項重要貢獻:通過使用具有單獨優化對比度采集參數的初步運動掃描,可精確估計變形場,使該方法具備模塊化特點。因此,MRF掃描可僅針對定量參數估計進行優化,無需考慮變形場估計問題。從應用角度來看,本研究為在極少被研究的區域(如呼吸肌,尤其是肋間肌和膈肌)中實現FF和𝑇1𝐻2𝑂的3D聯合量化奠定了基礎。
Abatract
摘要
Over the past decade, Magnetic Resonance Fingerprinting (MRF) has emerged as an efficient paradigm forthe rapid and simultaneous quantification of multiple MRI parameters, including fat fraction (FF), water T1(𝑇 1𝐻2𝑂), water T2 (𝑇 2𝐻2𝑂), and fat T1 (𝑇 1𝑓 𝑎𝑡). These parameters serve as promising imaging biomarkers invarious anatomical targets such as the heart, liver, and skeletal muscles. However, measuring these parametersin the upper body poses challenges due to physiological motion, particularly respiratory motion. In this work,we propose a novel approach, motion-corrected (MoCo) MRF T1-FF, which estimates the motion field using anoptimized preliminary motion scan and uses it to correct the MRF acquisition data before dictionary search forreconstructing motion-corrected FF and 𝑇 1𝐻2𝑂 parametric maps of the upper-body region. We validated thisframework using an in vivo dataset comprising 18 healthy volunteers (12 men, 6 women, mean age = 40 ± 14years old) and a 3 subjects with different neuromuscular disorders. At the ROI level, in regions minimallyaffected by motion, no significant bias was observed between the uncorrected and MoCo reconstructions forFF (mean difference of -0.6%) and 𝑇 1𝐻2𝑂 (?5.5 ms) values. Moreover, MoCo MRF T1-FF significantly reducedthe standard deviations of distributions assessed in these regions, indicating improved precision. Notably, inregions heavily affected by motion, such as respiratory muscles, liver, and kidneys, the MRF parametric mapsexhibited a marked reduction in motion blurring and streaking artifacts after motion correction. Furthermore,the diaphragm was consistently discernible on parametric maps after motion correction. This approach laysthe groundwork for the joint 3D quantification of FF and 𝑇 1𝐻2𝑂 in regions that are rarely studied, such as therespiratory muscles, particularly the intercostal muscles and diaphragm.
在過去十年中,磁共振指紋成像(MRF)已成為一種高效的成像范式,能夠快速且同步地量化多種MRI參數,包括脂肪分數(FF)、水的T1值(𝑇1𝐻2𝑂)、水的T2值(𝑇2𝐻2𝑂)以及脂肪的T1值(𝑇1𝑓𝑎𝑡)。這些參數在心臟、肝臟和骨骼肌等多種解剖部位中均是極具潛力的影像生物標志物。然而,由于生理運動(尤其是呼吸運動)的影響,在上半身測量這些參數面臨諸多挑戰。 ? 在本研究中,我們提出了一種新穎的運動校正(MoCo)MRF T1-FF方法。該方法通過優化的初步運動掃描估計運動場,并在字典搜索重建上半身區域的運動校正后脂肪分數(FF)和水的T1值(𝑇1𝐻2𝑂)參數圖之前,利用該運動場對MRF采集數據進行校正。我們使用包含18名健康志愿者(12名男性、6名女性,平均年齡為40±14歲)和3名不同神經肌肉疾病患者的在體數據集對該框架進行了驗證。 ? 在感興趣區域(ROI)層面,在受運動影響最小的區域中,未校正重建與運動校正重建的脂肪分數(FF)值(平均差異為-0.6%)和水的T1值(𝑇1𝐻2𝑂)(平均差異為-5.5 ms)之間未觀察到顯著偏差。此外,運動校正MRF T1-FF顯著降低了這些區域中測量值分布的標準差,表明精度得到改善。值得注意的是,在呼吸肌、肝臟和腎臟等受運動嚴重影響的區域,運動校正后MRF參數圖的運動模糊和條紋偽影顯著減少。而且,運動校正后的參數圖上能清晰識別膈肌。 ? 該方法為在呼吸肌(尤其是肋間肌和膈肌)等極少被研究的區域中實現脂肪分數(FF)和水的T1值(𝑇1𝐻2𝑂)的聯合3D量化奠定了基礎。
Method
方法
2.1. MR acquisition framework
The MR acquisition framework comprised two separate scans: firstly,a 3D radial stack-of-stars Fast Low Angle Shot (FLASH) sequence, referred to as the ‘‘motion scan’’, was used for estimating the deformationfields between the different respiratory phases. This was followed bythe 3D MRF T1-FF sequence, as proposed in Marty et al. (2021a) anacquisition specifically designed for joint estimation of 𝑇 1𝐻2𝑂, FF, B0,B1 and 𝑇 1𝐹 𝑎𝑡, to monitor muscle tissue alterations in subjects withneuromuscular diseases. The MRF T1-FF sequence consists of 1400golden angle radial spokes, incorporating varying echo times and flipangles allowing the reconstruction of a time-series of 175 undersampledimages at different contrasts (Marty and Carlier, 2020). The 3D MRFT1-FF sequence follows this principle, acquiring partitions sequentiallyto form a time-series of 175 3D volumes using a golden angle stack-ofstars radial sampling scheme (Feng, 2022). It can also be undersampledin the partition direction to accelerate the acquisition (Marty et al.,2021a).Navigators were inserted every 28 spokes within both sequences totrack the lung/liver interface during acquisition with a sampling frequency of approximately 10 Hz. These 1D gating spokes were centeredat a TE of 2.39 ms, to put water and fat signals in phase, therebymaximizing the liver signal and thus the contrast between the lungand liver. These navigators were acquired in the head-foot direction,with the readout center positioned around the dome of the liver anda field of view of 25 cm. A slice-selective RF pulse was applied with aslice thickness of 3 cm to obtain high signal-to-noise ratio and sufficientcontrast by minimizing the amount of subcutaneous fat projected ontothe navigator image. This parameter was empirically optimized byvisually analyzing the sharpness of the lung/liver interface on thenavigator images as a function of slice thickness in healthy volunteers.The 3D MRF T1-FF sequence diagram is provided in Fig. S1.
2.1. 磁共振采集框架 ? 磁共振采集框架包含兩次獨立掃描:首先,采用3D徑向堆疊星狀快速小角度激發(FLASH)序列(簡稱“運動掃描”),用于估計不同呼吸時相間的變形場。隨后,采用Marty等人(2021a)提出的3D MRF T1-FF序列——這是一種專為聯合估計水的T1值(𝑇1𝐻2𝑂)、脂肪分數(FF)、B0、B1和脂肪的T1值(𝑇1𝐹𝑎𝑡)設計的采集序列,旨在監測神經肌肉疾病患者的肌肉組織變化。 ? MRF T1-FF序列包含1400個黃金角徑向輻條,通過整合不同的回波時間和翻轉角,能夠重建175幅不同對比度的欠采樣圖像時間序列(Marty和Carlier,2020)。3D MRF T1-FF序列遵循這一原理,采用黃金角堆疊星狀徑向采樣方案依次采集分區,形成包含175個3D體積的時間序列(Feng,2022)。為加快采集速度,該序列還可在分區方向進行欠采樣(Marty等人,2021a)。 ? 在兩個序列中,每28個輻條插入一個導航器,以約10 Hz的采樣頻率在采集過程中追蹤肺/肝界面。這些1D門控輻條的回波時間(TE)中心設為2.39 ms,使水和脂肪信號處于同相狀態,從而最大化肝臟信號,增強肺與肝之間的對比度。導航器沿頭-足方向采集,讀出中心位于肝臟頂部附近,視野為25 cm。施加層選擇性射頻脈沖,層厚為3 cm,通過減少投射到導航器圖像上的皮下脂肪量,以獲得高信噪比和足夠的對比度。該參數是通過在健康志愿者中視覺分析不同層厚下導航器圖像上肺/肝界面的清晰度,經經驗優化確定的。3D MRF T1-FF序列示意圖見圖S1。
Conclusion
結論
In this study, we introduced a modular acquisition approach comprising a preliminary motion scan for estimating deformation fields,followed by an MRF scan for quantifying multiple parameters, accompanied by a reconstruction framework for iteratively reconstructing motion-corrected MRF singular volumes. This methodology significantly mitigated motion blurring and streaking artifacts in resulting3D 𝑇 1𝐻2𝑂 and FF parametric maps with a spatial resolution of 1𝑥1 𝑥 5 mm3 , leading to marked improvements in precision. Consequently, accurate detection and delineation of moving structureswas consistently achieved, with measured values aligning closely withthose reported in the literature. These advancements pave the way forquantifying FF and 𝑇 1𝐻2𝑂 in regions previously challenging to assessdue to motion, such as the respiratory muscles.
在本研究中,我們提出了一種模塊化采集方法,該方法包括用于估計形變場的初步運動掃描、用于量化多參數的MRF掃描,以及用于迭代重建運動校正后MRF奇異容積的重建框架。此方法顯著減輕了空間分辨率為1×1×5 mm3的3D 𝑇1𝐻2𝑂和FF參數圖中的運動模糊與條狀偽影,大幅提升了測量精度。因此,我們能夠穩定實現對運動結構的準確檢測與清晰勾勒,且測量值與文獻報道結果高度一致。這些進展為量化此前因運動干擾而難以評估的區域(如呼吸肌)的FF和𝑇1𝐻2𝑂值奠定了基礎。
Results
結果
The total processing time per patient was approximately 7 h, including over 3 h for training the neural network on the input motion scanvolumes for deformation field estimation, which, at this stage, was donefor every dataset. The training used around 44 gigabits of GPU memory.The difference images between the reference respiratory phase andthe other phases, with and without applying registration, show the effectiveness of the displacement field estimation on the motion scan (2)(Fig. 4). Supplementary videos (Fig.S4) demonstrating the registrationresults are also available for one healthy control. In the uncorrectedimages, noticeable movements included the liver descending from fullexpiration to full inspiration, and the abdominal ribcage shifting tothe right. However, in the registered images, differences against thereference phase were greatly reduced for all motion states. The meanand standard deviation of SSIM and NRMSE across all subjects andslices were calculated and reported in Table 1. The mean NRMSEof respiratory phases 2, 3, 4, and 5 against the reference respiratoryphase 1 decreased from 0.10, 0.20, 0.28, and 0.33 to 0.04, 0.06, 0.07,and 0.08, respectively. Similarly, the mean SSIM index increased from98.6%, 96.3%, 94.2%, and 93.2% to 99.8%, 99.6%, 99.5%, and 99.3%,respectively. In contrast, MRF singular volumes suffered from highartefacts and contrast variation across respiratory phases, making themunsuitable for direct deformation field estimation as in Cruz et al.(2022) (Fig. S5 and S6).
每位患者的總處理時間約為7小時,其中包括超過3小時用于在輸入的運動掃描容積上訓練神經網絡以估計形變場——在當前階段,每個數據集都需要執行這一步驟。訓練過程約占用44吉比特的GPU內存。 ? 參考呼吸相位與其他相位的差異圖像(無論是否應用配準)顯示,運動掃描的位移場估計具有有效性(圖4)。補充視頻(圖S4)也展示了一名健康對照者的配準結果。在未校正的圖像中,可觀察到明顯的運動現象,包括肝臟從完全呼氣狀態到完全吸氣狀態時的下移,以及腹部胸腔向右偏移。然而,在配準后的圖像中,所有運動狀態下與參考相位的差異均大幅減小。 ? 所有受試者和所有層面的結構相似性(SSIM)及歸一化均方根誤差(NRMSE)的均值與標準差已計算并在表1中呈現。呼吸相位2、3、4、5相對于參考呼吸相位1的平均NRMSE分別從0.10、0.20、0.28、0.33降至0.04、0.06、0.07、0.08;同樣,平均SSIM指數分別從98.6%、96.3%、94.2%、93.2%提升至99.8%、99.6%、99.5%、99.3%。 ? 相比之下,MRF奇異容積存在嚴重的偽影,且不同呼吸相位間的對比度差異較大,因此不適合采用如Cruz等人(2022年)所述的直接形變場估計方法(圖S5和S6)。
Figure
圖
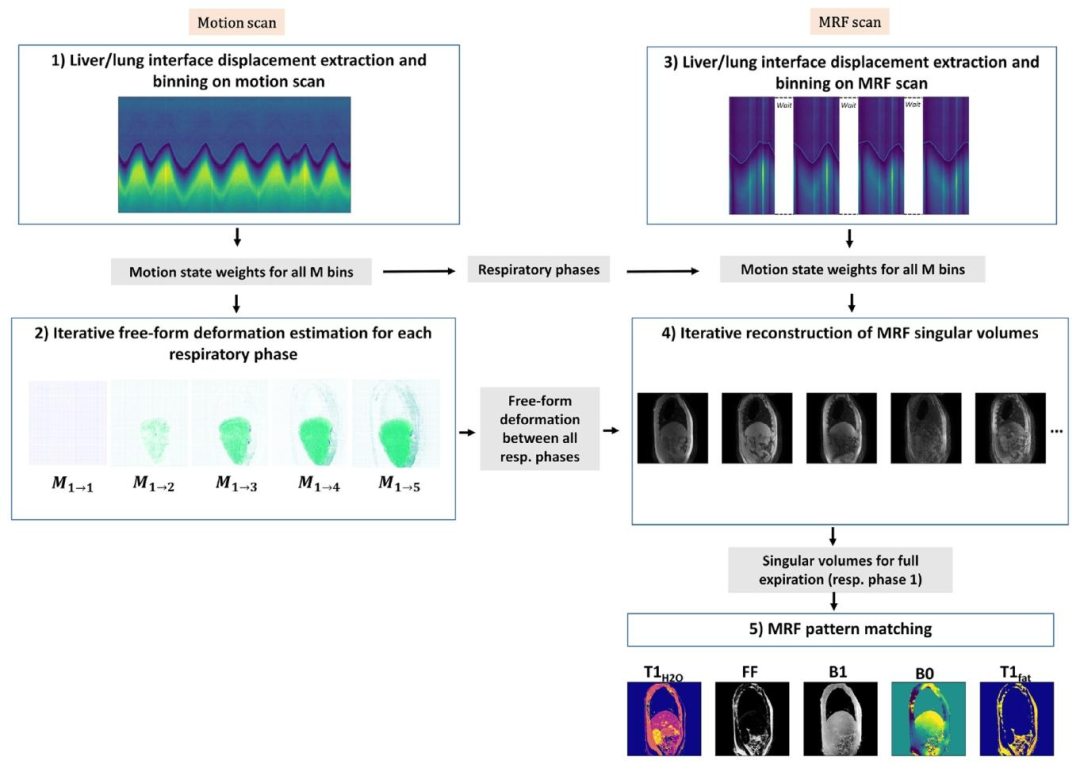
Fig. 1. Diagram illustrating the different steps of the proposed reconstruction framework for reconstructing the motion-corrected parametric maps: (1) Liver/lung interfacedisplacement extraction and binning on the motion scan (Section 2.2.1); (2) Iterative free-form deformation estimation on the motion scan volumes for each respiratory phase(Section 2.2.2); (3) Liver/lung interface displacement extraction and binning on the MRF scan (Section 2.2.1); (4) Iterative reconstruction of MRF singular volumes using thedeformation fields estimated in step 2 (Section 2.2.3); (5) MRF pattern matching using bi-component dictionary matching, as proposed in Slioussarenko et al. (2024), to reconstruct𝑇1𝐻2𝑂, FF, B0, B1 and 𝑇 1𝐹 𝑎𝑡 parametric maps from the motion-corrected singular volumes.
圖1. 所提出的運動校正參數圖重建框架各步驟示意圖: ? (1)從運動掃描中提取肝/肺界面位移并進行分箱處理(詳見2.2.1節); ? (2)在運動掃描的各呼吸時相體積上迭代估計自由形式變形(詳見2.2.2節); ? (3)從MRF掃描中提取肝/肺界面位移并進行分箱處理(詳見2.2.1節); ? (4)利用步驟2中估計的變形場迭代重建MRF單個體積(詳見2.2.3節); ? (5)采用Slioussarenko等人(2024)提出的雙組分字典匹配法進行MRF模式匹配,從運動校正后的單個體積中重建水的T1值(𝑇1𝐻2𝑂)、脂肪分數(FF)、B0、B1和脂肪的T1值(𝑇1𝐹𝑎𝑡)參數圖。
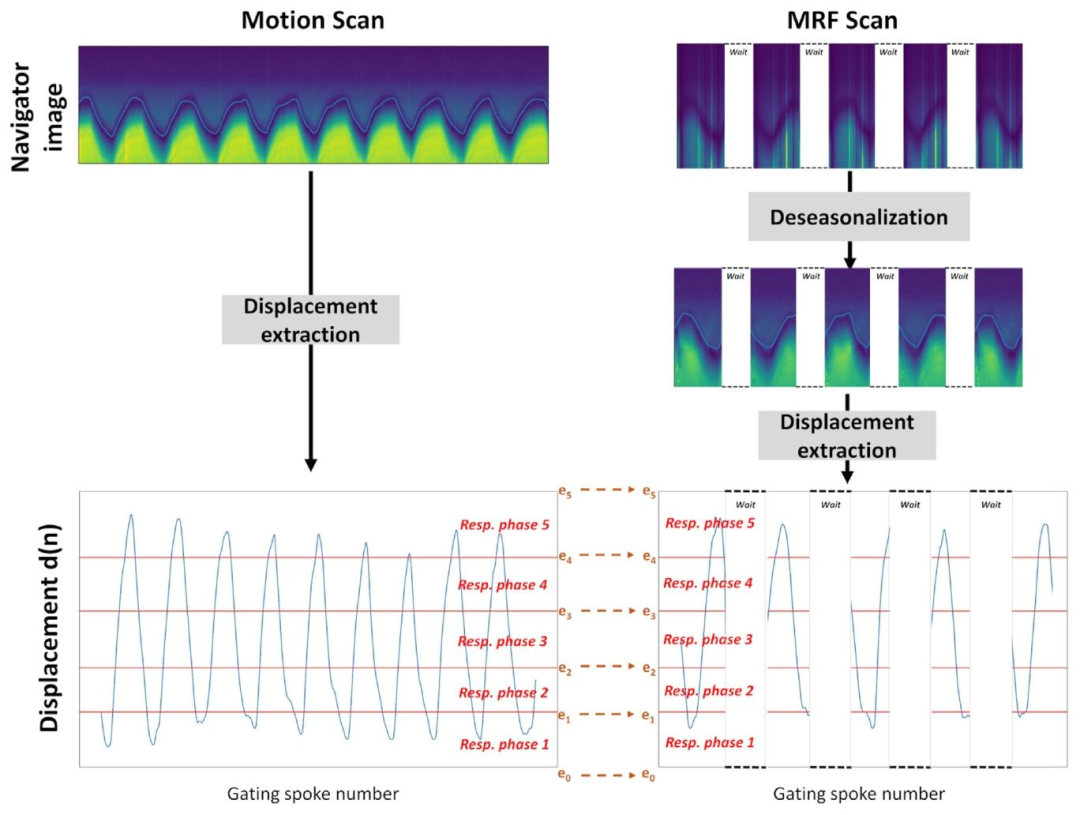
Fig. 2. Representative examples of the liver/lung interface displacement estimation d(n), along with data binning, derived from both motion and 3D MRF T1-FF scans usingnavigator images. The navigator images for the 3D MRF-T1-FF scan undergo a deseasonalization procedure initially to eliminate the seasonal MRF component. The z-coordinatesof each respiratory phases ([𝑒0 , … , 𝑒𝑀 ]) are determined from the motion scan data to ensure an equal number of spokes per respiratory phase. These coordinates were then appliedto bin the MRF scan data. The jumps on the MRF navigator images are due to the fact that there is a recovery time at the end of each repetition to allow for the magnetizationto grow back to equilibrium
圖2. 利用導航器圖像從運動掃描和3D MRF T1-FF掃描中提取的肝/肺界面位移估計值d(n)及數據分箱的代表性示例 ? 3D MRF-T1-FF掃描的導航器圖像首先經過去季節性處理,以消除MRF的季節性成分。各呼吸時相([𝑒0,…,𝑒𝑀])的z坐標由運動掃描數據確定,以確保每個呼吸時相包含相同數量的輻條。這些坐標隨后被用于對MRF掃描數據進行分箱。MRF導航器圖像上的跳變源于每次重復結束時存在恢復時間,以使磁化強度恢復至平衡狀態。
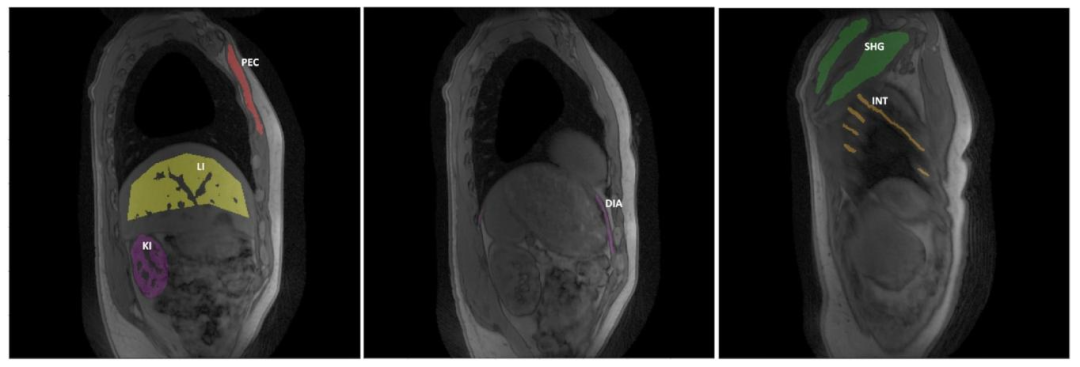
Fig. 3. Illustration of the pole ladder adapted from Lorenzi and Pennec (2014) fortransporting a single deformation between templates 𝑇0 and 𝑇**𝑖 to estimate the stationaryvelocity field (SVF) connecting the reference individual image 𝐼0 and the predictedindividual image 𝐼**𝑖 ′ . Steps include obtaining longitudinal SVF between templates (𝒖),SVF between template and individual image (𝒗), and deriving diffeomorphisms forparallel transport. The resulting parallel transported deformation is obtained throughthe conjugate actions of diffeomorphisms parameterized by SVFs 𝒖 and 𝒗. A truncatedBaker-Campbell-Hausdorff (BCH) formula is used for computations (denotes as 𝛱), anda scaling factor 𝑛 ensures computational efficiency
圖3. 基于Lorenzi和Pennec(2014)改編的“極點階梯”示意圖,用于在模板𝑇?和𝑇?之間傳輸單一變形,以估計連接參考個體圖像𝐼?和預測個體圖像𝐼?'的靜止速度場(SVF)。 ? 步驟包括:獲取模板間的縱向SVF(𝒖)、模板與個體圖像間的SVF(𝒗),以及推導用于平行傳輸的微分同胚。最終的平行傳輸變形通過由SVF 𝒖和𝒗參數化的微分同胚的共軛作用獲得。計算中使用截斷的貝克-坎貝爾-豪斯多夫(BCH)公式(記為𝛱),縮放因子𝑛確保計算效率。
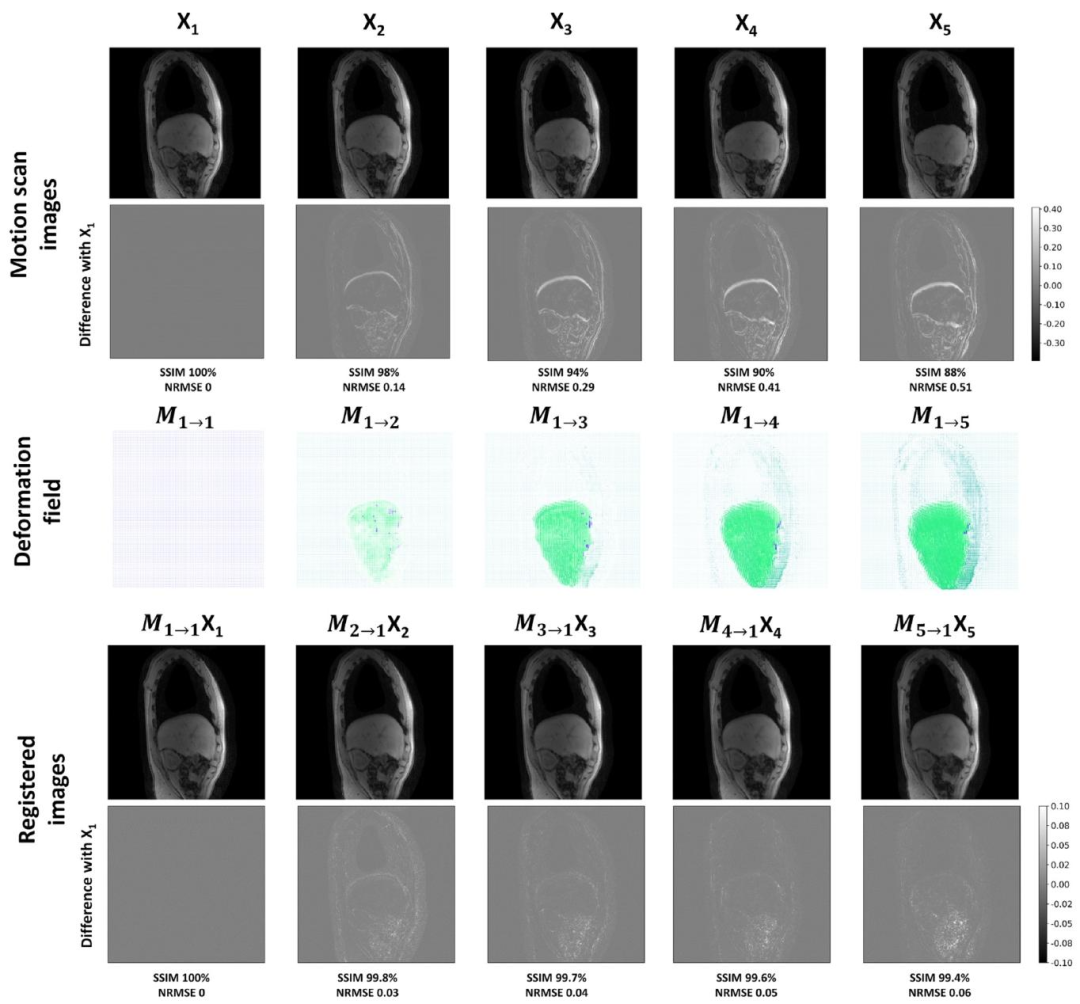
Fig. 4. Deformation field estimation and registration accuracy. The top two rows depict images reconstructed from the motion scan before registration, along with the differencecompared to the reference full expiration respiratory phase (phase 1) for a single sagittal slice in a healthy subject. The third row displays the deformation fields estimated usingVoxelMorph, while the last two rows illustrate the resulting difference images between the reference respiratory phase volume and the registered volume.
圖4. 變形場估計與配準精度示例 ? 最上方兩行展示健康受試者單個矢狀切片在配準前的運動掃描重建圖像,以及與參考完全呼氣呼吸時相(時相1)的差異圖像。第三行顯示通過VoxelMorph估計的變形場,最后兩行則呈現參考呼吸時相體積與配準后體積的差異圖像。
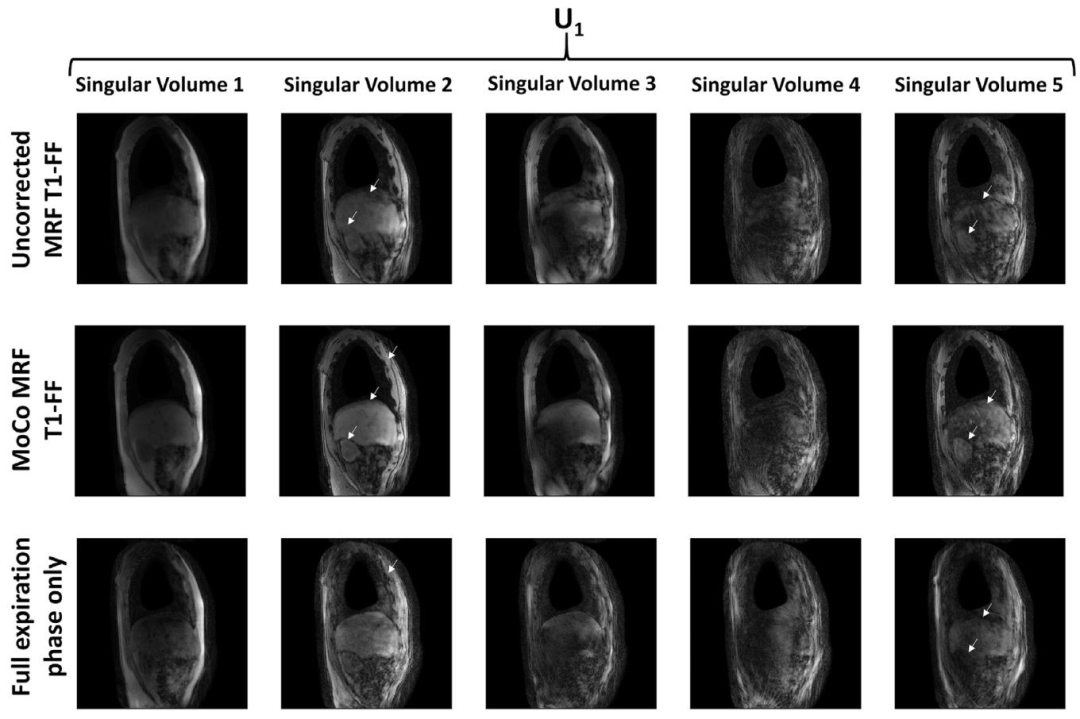
Fig. 5. The first five singular volumes reconstructed from the uncorrected MRF T1-FF sequence reconstructing the singular volumes from a nuFFT on all acquired data withoutbinning or motion correction, the motion-corrected (MoCo) MRF T1-FF data, and from the MRF T1-FF data acquired during the reference respiratory phase only (correspondingto the full-expiration phase). White arrows highlight areas where motion blurring, undersampling artefacts, and weak contrast are notably reduced following motion correction.
圖5. 前五幅奇異容積分別通過以下方式重建:未校正的MRF T1-FF序列(基于所有采集數據的nuFFT重建,未進行分箱或運動校正)、運動校正(MoCo)后的MRF T1-FF數據,以及僅在參考呼吸相位(對應完全呼氣相位)采集的MRF T1-FF數據。白色箭頭標注的區域顯示,經過運動校正后,運動模糊、欠采樣偽影和對比度不足的問題得到顯著改善。
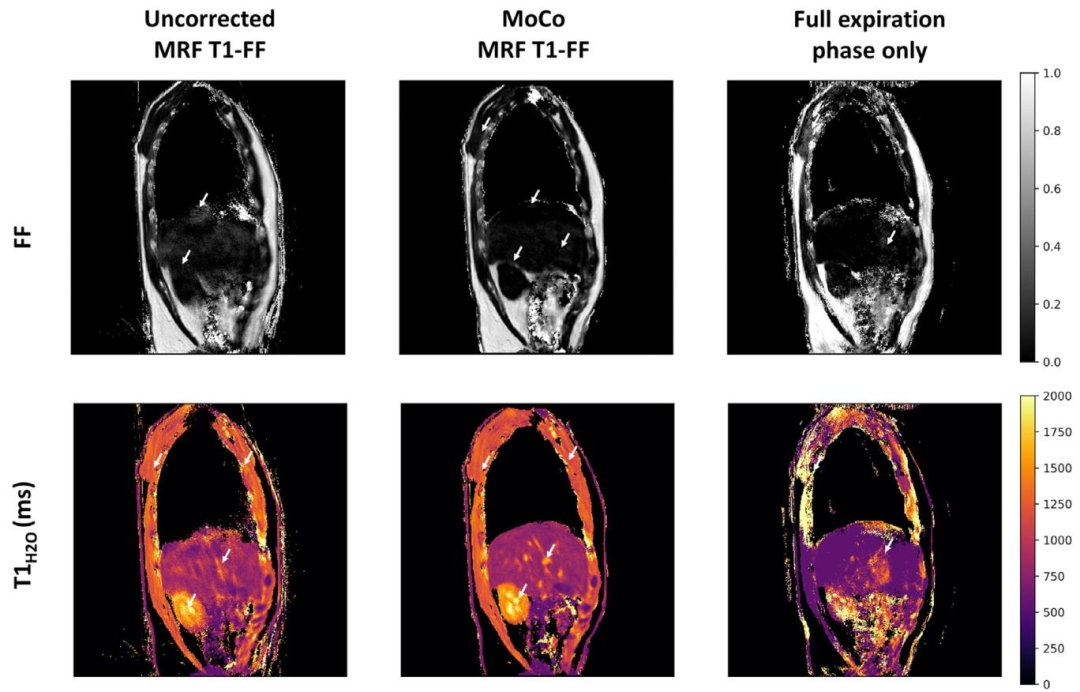
Fig. 6. Representative parametric FF and 𝑇 1𝐻2𝑂 maps obtained at one slice level in a healthy volunteer derived from the uncorrected and motion-corrected (MoCo) MRF T1-FFreconstructions, and from the MRF T1-FF data acquired during the reference respiratory phase only (corresponding to the full-expiration phase). White arrows emphasize regionswhere motion blurring, parameter estimation bias and streaking artifacts are significantly mitigated with the motion correction method.
圖6 健康志愿者某一層面的代表性參數FF圖和𝑇1𝐻2𝑂圖,這些圖像分別來源于未校正的MRF T1-FF重建結果、運動校正(MoCo)后的MRF T1-FF重建結果,以及僅在參考呼吸相位(對應完全呼氣相位)采集的MRF T1-FF數據。白色箭頭標注的區域顯示,運動校正方法顯著減輕了運動模糊、參數估計偏差和條狀偽影問題。
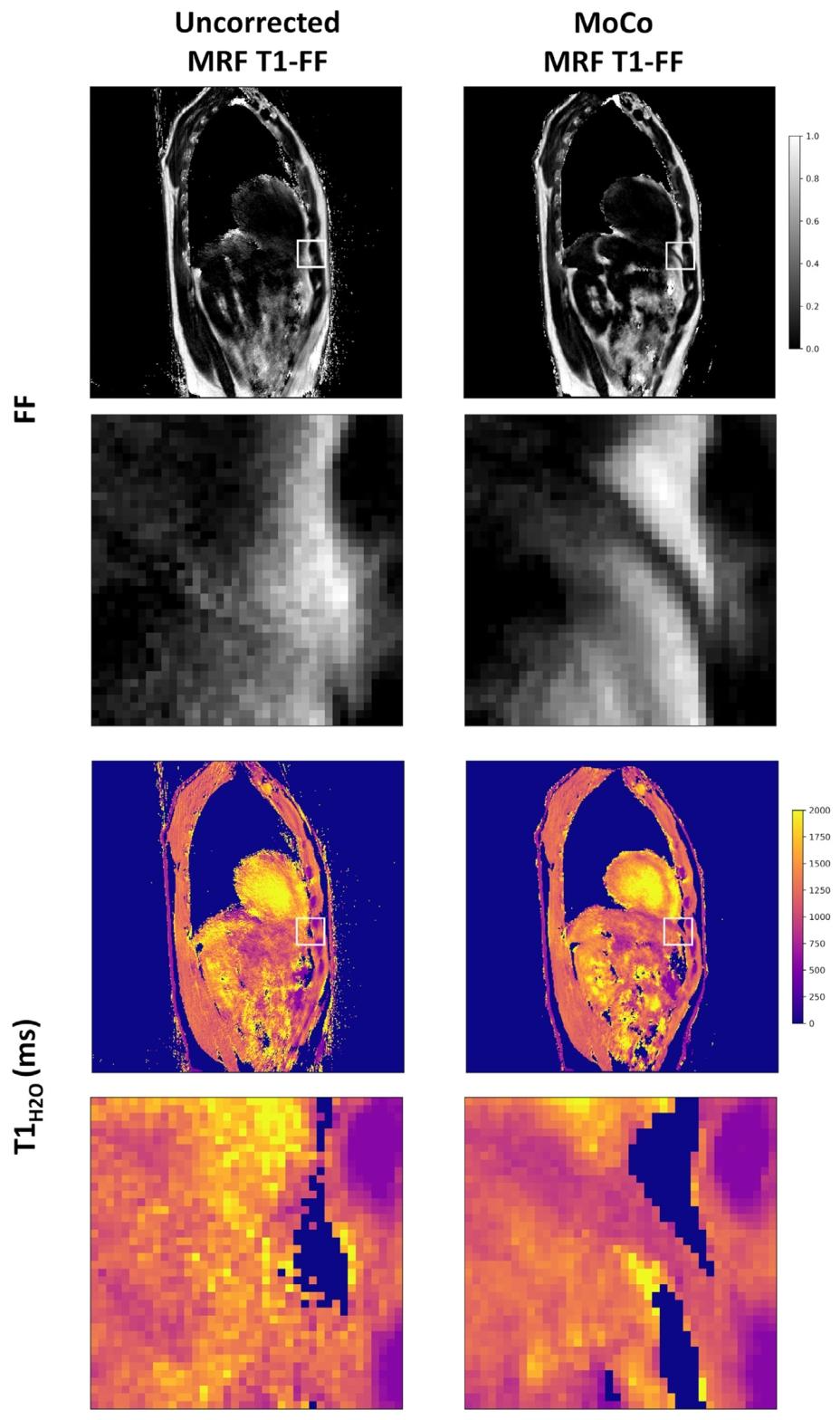
Fig. 7. Representative parametric FF and *𝑇* 1*𝐻*2*𝑂* maps obtained at one slice level in a healthy volunteer derived from both the uncorrected and motion-corrected (MoCo) MRFT1-FF reconstructions. The second and fourth rows present zoomed images corresponding to the white squares on the full images. Following motion correction, a thin layer ofmuscle is discernible on the FF and 𝑇 1𝐻2𝑂maps, corresponding to the diaphragm
圖7 健康志愿者某一層面的代表性參數FF圖和𝑇1𝐻2𝑂圖,這些圖像分別來源于未校正的MRF T1-FF重建結果和運動校正(MoCo)后的MRF T1-FF重建結果。第二行和第四行為全圖中白色方框區域對應的放大圖像。運動校正后,在FF圖和𝑇1𝐻2𝑂圖上可清晰辨別出一層薄薄的肌肉組織,該組織對應膈肌。?
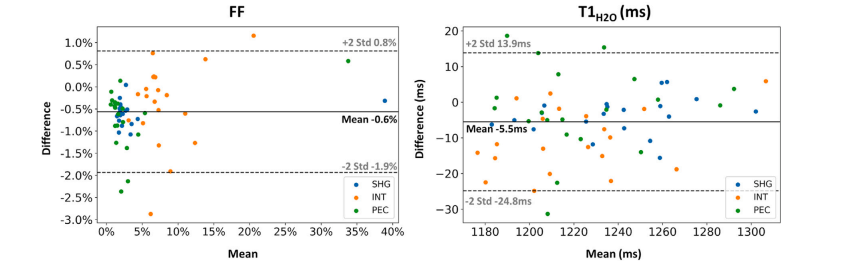
Fig. 8. Bland–Altman plot comparing the ROI-level fat fraction (FF) and water T1 (𝑇 1𝐻2𝑂) values derived from both the uncorrected and motion-corrected (MoCo) MRF T1-FFreconstruction methods in the shoulder girdle (SHG), intercostal muscles (INT), and Pectoralis major (PEC). Each data point represents an individual subject. The solid line representsthe mean difference between the values obtained with both reconstructions, while the dashed lines indicate the 95% confidence interval. Data points for FF and subject 20 withan unlabeled myopathy were excluded from this plot as the FF maps with no motion correction were highly artefacted, with fat-water swaps.
圖8 ?Bland–Altman圖比較了在肩胛帶(SHG)、肋間肌(INT)和胸大肌(PEC)區域中,由未校正和運動校正(MoCo)的MRF T1-FF重建方法得到的感興趣區(ROI)脂肪分數(FF)和水T1(𝑇1𝐻2𝑂)值。每個數據點代表一名受試者。實線表示兩種重建方法所得數值之間的平均差異,虛線表示95%置信區間。由于未進行運動校正的FF圖存在嚴重偽影(伴隨脂肪-水信號互換),本圖中排除了FF的相關數據點以及患有未標注肌病的20號受試者的數據。
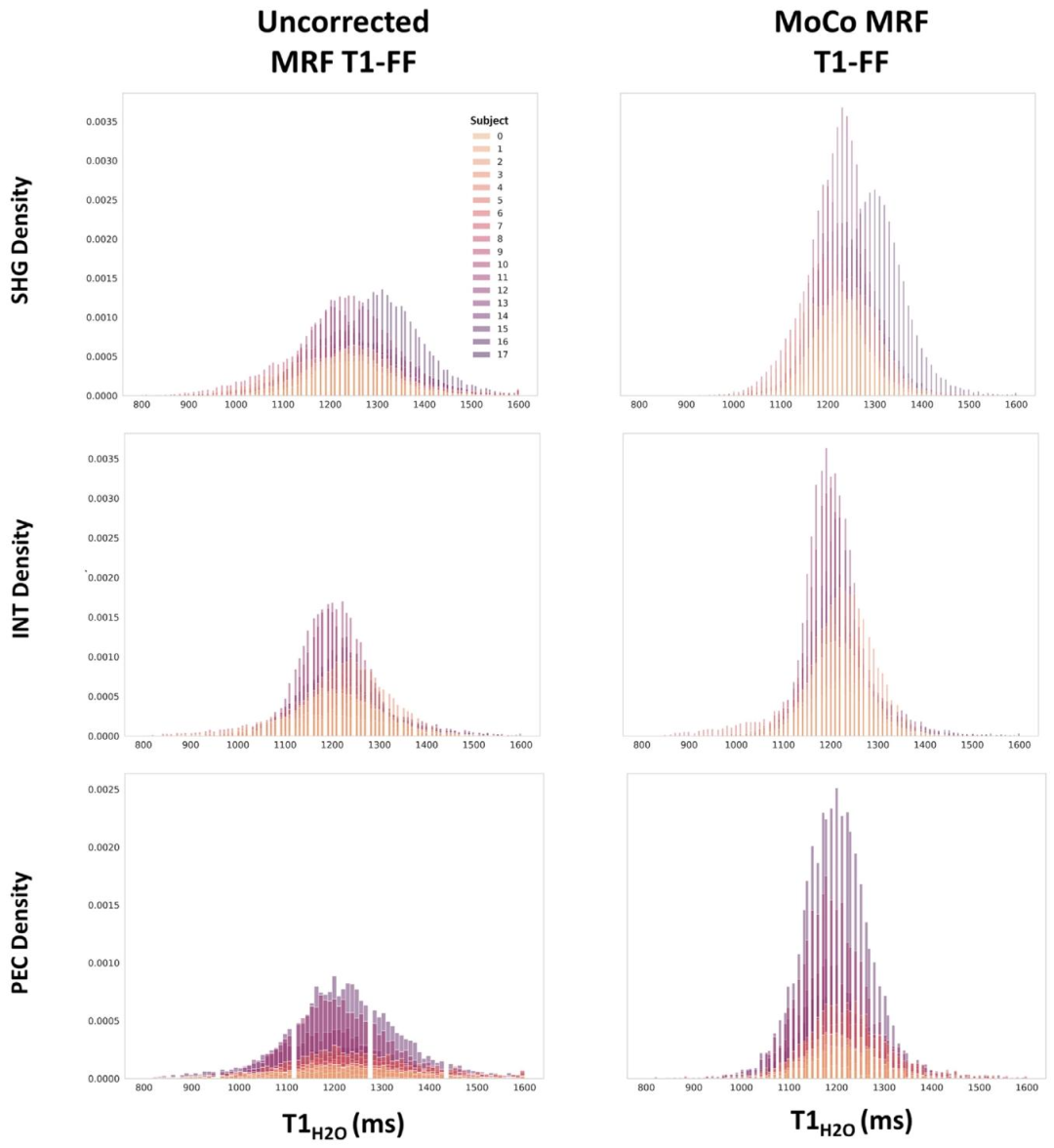
Fig. 9. Distribution of fat fraction (FF) and water T1 (𝑇 1𝐻2𝑂) values obtained in the shoulder girdle (SHG), intercostal muscles (INT), and Pectoralis major (PEC) from theuncorrected and the motion-corrected (MoCo) MRF T1-FF reconstruction. Each color represents an individual subject
圖9 肩胛帶(SHG)、肋間肌(INT)和胸大肌(PEC)區域中,由未校正和運動校正(MoCo)的MRF T1-FF重建方法得到的脂肪分數(FF)和水T1(𝑇1𝐻2𝑂)值的分布。不同顏色代表不同的受試者。
Table
表
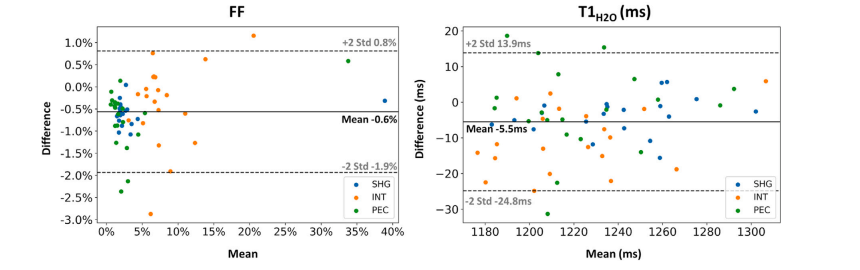
Table 1Structural similarity (SSIM) and normalized root mean square error (NRMSE) between the volumes of respiratory phases2–5 and reference respiratory phase (respiratory phase 1, corresponding to full expiration) for all subjects before andafter motion registration. Data are presented as mean ± standard deviation
表1 所有受試者在運動配準前后,呼吸相位2–5與參考呼吸相位(呼吸相位1,對應完全呼氣狀態)的容積之間的結構相似性(SSIM)和歸一化均方根誤差(NRMSE)。數據以平均值±標準差表示 。
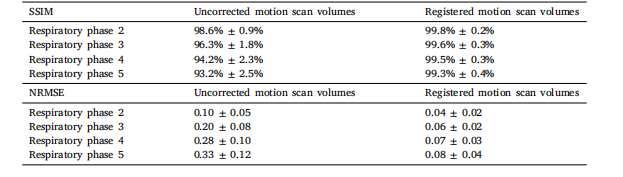
Table 2ROI-level fat fraction (FF) and water T1 (𝑇 1𝐻2𝑂) values (mean ± standard deviation) measured in the 18 healthy volunteers (Subjects 0-17) and 3subjects with DMD, IBM and unlabeled myopathy (Subjects 18-20) for the uncorrected and motion-corrected (MoCo) MRF T1-FF reconstructionsassessed in the shoulder girdle (SHG), intercostal muscles (INT), and Pectoralis major (PEC) muscles. Illustrative literature values were addedin the last row of each table section
表2 ?在肩胛帶(SHG)、肋間肌(INT)和胸大肌(PEC)區域中,對18名健康志愿者(0-17號受試者)以及3名分別患有杜氏肌營養不良癥(DMD)、包涵體肌炎(IBM)和未標注肌病的受試者(18-20號受試者),采用未校正和運動校正(MoCo)的MRF T1-FF重建方法測得的感興趣區(ROI)脂肪分數(FF)和水T1(𝑇1𝐻2𝑂)值(平均值±標準差)。每個表格部分的最后一行添加了示例性的文獻參考值。
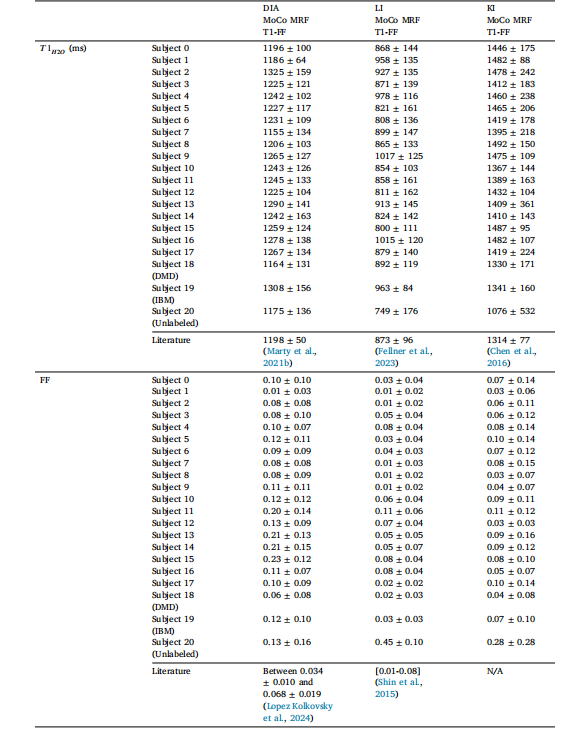
Table 3ROI-level fat fraction (FF) and water T1 (𝑇 1𝐻2𝑂) values (mean ± standard deviation) measured in the 18 healthy volunteers (Subjects 0-17) and 3subjects with DMD, IBM and unlabeled myopathy (Subjects 18-20) for the uncorrected and motion-corrected (MoCo) MRF T1-FF reconstructionsassessed in the diaphragm (DIA), kidneys (KI) and liver (LI). Illustrative literature values were added in the last row of each table section
表3 ?在膈肌(DIA)、腎臟(KI)和肝臟(LI)區域中,對18名健康志愿者(0-17號受試者)以及3名分別患有杜氏肌營養不良癥(DMD)、包涵體肌炎(IBM)和未標注肌病的受試者(18-20號受試者),采用未校正和運動校正(MoCo)的MRF T1-FF重建方法測得的感興趣區(ROI)脂肪分數(FF)和水T1(𝑇1𝐻2𝑂)值(平均值±標準差)。每個表格部分的最后一行添加了示例性的文獻參考值。


Phantom-Data:邁向通用的主體一致性視頻生成數據集)


)


)
--(C/C++))
力扣.二叉樹的最大路徑和牛客.主持人調度(二))
:標準IO高級操作與文件流定位實戰》)




)
)

